
| Ionic | Covalent | Metallic |
|---|---|---|
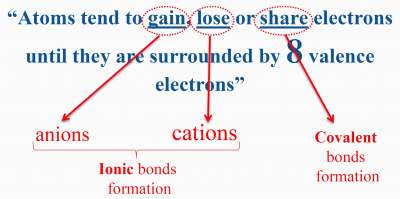
| Results from the electrostatic attraction and cations between and anions. In ionic compounds, electrons transfer from cations to anions. Example: NaCl |
Results from sharing of electrons between two non-metallic atoms.
In ionic compounds, electrons transfer from cations to anions. Exists mainly among non−metallic elements. Example: HCl |
Mainly found in metals, where the metalsbonding electrons are free to move throughout the 3D structure of the metal. Example: Na |
 |
 |
 |
 |