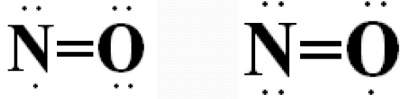Exceptions to Octet Rule and bf3 lewis structure
Reminder about the Octet Rule
"Atoms tend to gain, loseor shareelectrons until they are surrounded by 8 valence electrons"
Exceptions to the Octet Rule
- Atoms with a total odd number of electrons
- Less than Octet
- More than Octet
Example of Atoms with a total odd number of electrons: NO Molecule
Total number of valence electrons = 5 (of N) + 6 (of O) = 11 (odd number)
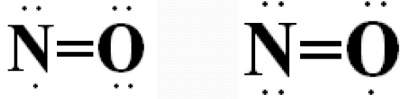
Example of Less than Octet: BF3 Molecule
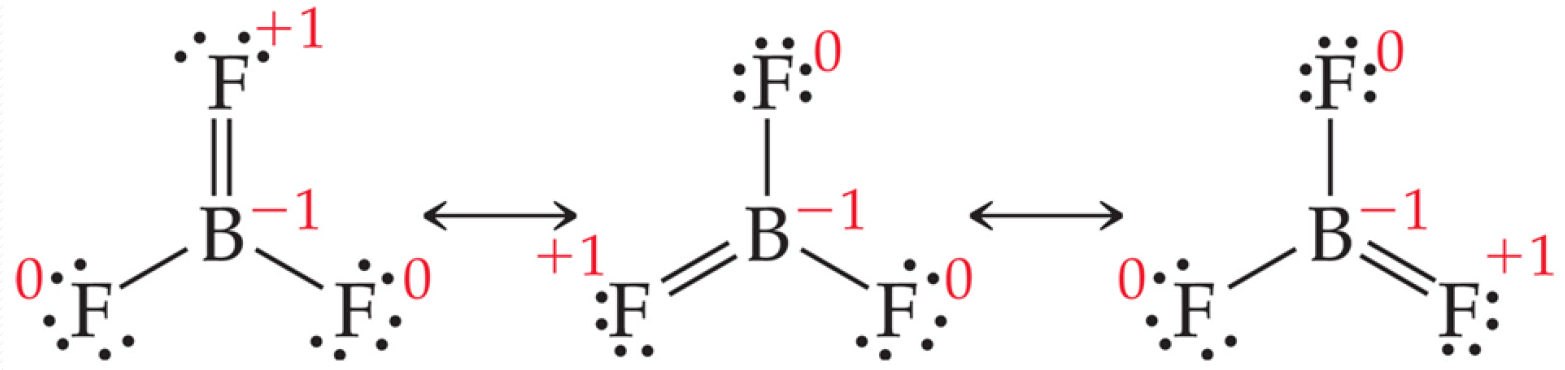
Despite these correct resonance structures, they are not possible due to the unacceptable formal charge on F.
Example of More than Octet: PCl5 Molecule
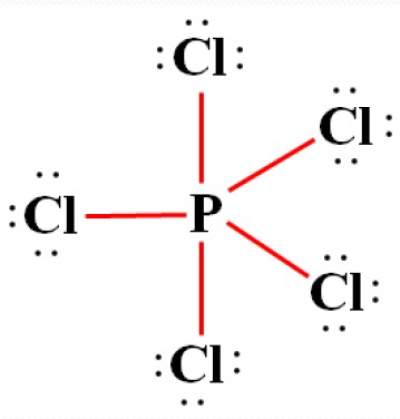
- Where the central atom (P) is surrounded by more than octet (8).
- This is observed in elements where the 3rd shell (and beyond) are being filled
- Here, the outermost configuration of P is: 3s2, 3p3, 3d0
- Such elements exceed octet by accommodating more electrons in their empty d orbitals
For more details, please contact me here.