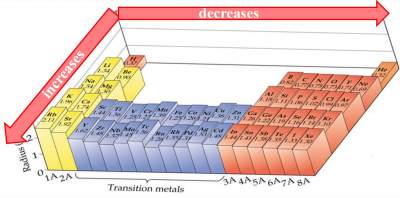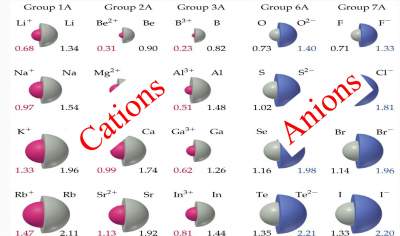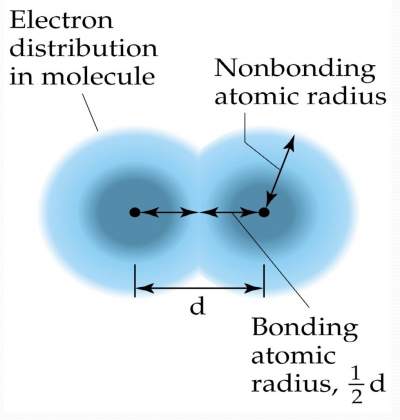

| As we move across the periodic table, the number of core electrons remains constant, while the nuclear charge increases. Hence, there is an increased attraction between the nucleus and the outermost electrons. This attraction causes the atomic radius to decrease. (The rule of Zeff) |  |
| As the principle quantum number (n) increases (by moving down a group), the distance of the outermost electron from the nucleus becomes larger, and the atomic radius increases.(The rule of n) | 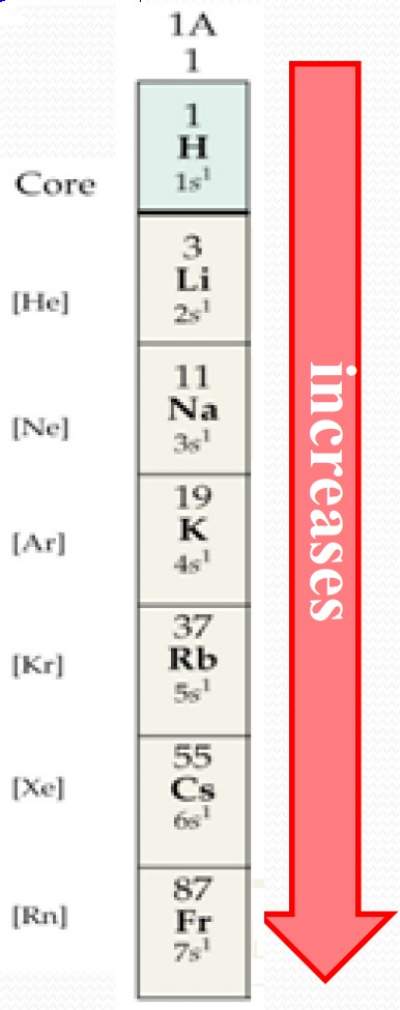 |