Periodic Table
Introduction
Elements are arranged according to an increasing atomic number with elements having similar properties placed in vertical columns.
It is used to organize the 114 elements in a meaningful way.
As a consequence of this organization, there are periodic properties associated with the periodic table as shown below:
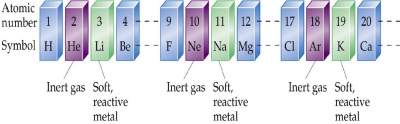
Numbering in the periodic table
The North American way and IUPAC (The International Union of Pure &Applied Chemistry) are the numbering methods as illustrated below:
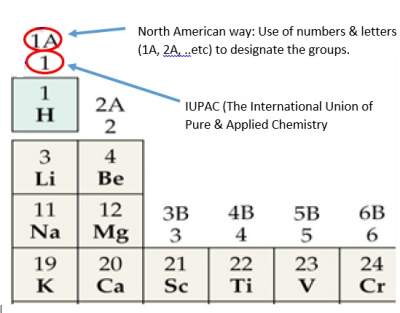
Columns in the periodictable are called groups(numbered from 1A to 8A or 1 to 18).
Rows in the periodic table are called periods (sometimes numbered from 1 to 7).
Atomic Weight
Fractions in the periodic table are important. For example 15.99O
- Atomic Mass = Number of Protons + Number of Neutrons
- Atomic Weight = average atomic mass, Which is calculated using different isotopes of the same element based on the relative (%) abundance this element.
Exercise: Find Atomic Weight
Exercise-1 on Atomic Weight
Check your answers here:
Solution to Exercise-1 on Atomic Weight
For more details, please contact me here.
Date of last modification: Summer , 2019

