Oxidation-Reduction Reactions
Oxidation
This is the charge over the atom or ion (cation or anion).
Examples:
| Atom | Oxidation Number | Ion | Oxidation Number |
| Na | 0 | Na+ | +1 |
| H | 0 | H+ | +1 |
| Cl | 0 | Cl− | − 1 |
| Fe | 0 | Fe2+ | +2 |
Remark: Some elements can have more than one oxidation number for example Fe
Rules of the Oxidation Numbers (ON)
- An atom in the elemental state has a ZERO oxidation number. Examples: Ca, H2, 42, S8
- The oxidation number of a monoatomic ion is the charge on the ion. Examples: (+1) for Na+, (− 2) for S2−, (3+) for Al3+
- Nonmetals have negative oxidation numbers, with some exceptions:
- O in ionic & molecular compounds has an ON of (-2) but inperoxides (such as H2O2),
its ON is (-1) (because the ion is O2+2−).
- H has an ON of (+1) with nonmetals and (-1) with metals.
- F has an ON of (-1) in all compounds. Other halogens can have ON of (-1) with most binary compounds except with O, they have ON of (+1)
- The sum of the ON of all atoms in a neutral molecule is ZERO.
Example: H2O; [2 ×(+1)] + [[1 ×(− 2)] = ZERO
However, the sum of the ON in a polyatomic ion equals the charge of the ion.
Example: H3O+
[3 × (+1)] + [1 × (-2)] = +1
Example: The Oxidation numbers of S in its compounds
- In S2: which is the element form of Sulfur
→ ONS is ZERO
- In H2S: This is a neutral molecule (net charge = zero) = [2 × (+1) ] + ONS
→ ONS = − 2
- In SCl2: This is a neutral molecule (net charge = zero) = ONS + [2 × (− 1)]
→ ONS = +2
- In Na2SO3:This is a neutral molecule (net charge = zero) = [2 × (+1) + ONS + [3 × (− 2)]] → ONS = +4
- In SO42−: This is an anion (net charge = − 2)
→ ONS = +6
→ ON of S can be: − 2, 0, +2, +4, +6 depending on its compounds.
Oxidation and Reduction Occuring Simultaneously
Remark: When a substance is oxidized, another should be reduced as illustrated below:
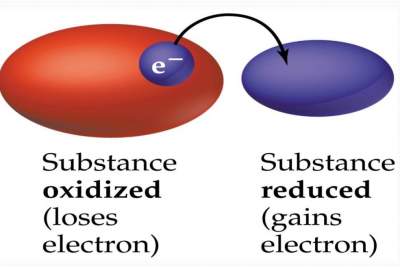
Question: How do we determine whether a chemical reaction is an oxidation-reduction reaction?
Answer:
By following the changes in the oxidation numbers of each element in the reaction.
Example
Zn(s) + 2HCl(l) → ZnCl2(aq) + H2(g)
Here, during oxidation, Zn(s) lost 2e to become Zn2+ whereas H+ gained 2e to become H2(g)
Zn (ON is zero) is oxidized to Zn2+ (ON is +2 ) , while H(ON is +1 ) is reduced to H2 (ON is zero).
H caused Zn to be oxidized → H is an "oxidixing agent"
Zn caused H to be reduced → Zn is an "reducing agent"
Balancing Equations
- Both mass and charge is reserved in chemical reactions.
- Electrons transfer from one compound to another, but not lost in a chemical reaction.
- A convenient way to balance an oxidation-reduction reaction is to separate the reaction into half reactions,
and deal with each half reaction separately then recombine them in a one final balanced reaction.
Examples
- Cr2O72−(aq) + CH3OH(aq) → Cr3+(aq) + HCO2H(aq)
- NO2−(aq) + Cr2O72−(aq) → Cr3+(aq) + NO3−(aq)
Balancing Equations in a Basic Solution
Here OH− and H2O are used instead of H+ and H2O
The same method is used, but here, OH− is added to "neutralize"
the H+ used, forming H2O
Examples:
- MnO4−(aq) + Br−(aq) → MnO2(s) + BrO3−(aq)
- Cr(OH)3(s) + ClO−(aq) → CrO42−(aq) + Cl2(g)
For more details, please contact me here.
Date of last modification: Summer , 2019
