Example:CH4, NH3 and H2O, all have the same electron-domain geometry; tetrahedral with different bond angles.
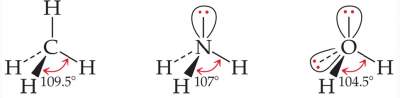
This is attributed to the repulsion between the high electron density in the non-bonding pairs and the close bonding pairs.
This results in different molecular geometries of these molecules.
Multiple Bonds
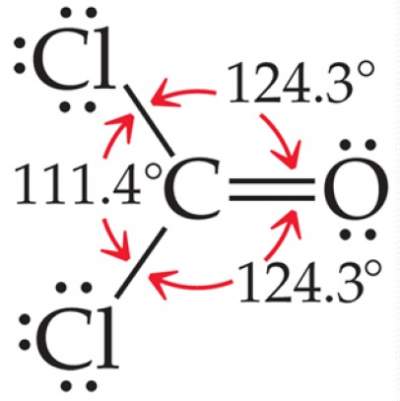
Multiple Bonds also affect bond angles in molecules containing them.Example:Cl2CO molecule
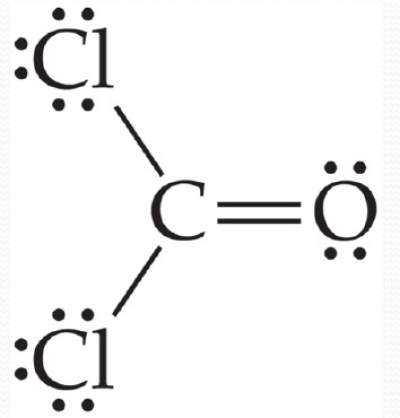
The central atom is surrounded by 3 bonding electron domains, hence both the electron-domain and molecular geometries should be:
Trigonal Planarwith a bond angle of 120°