Bond dipoles DO NOT cancel each other.
So, H2O is a POLAR molecule.
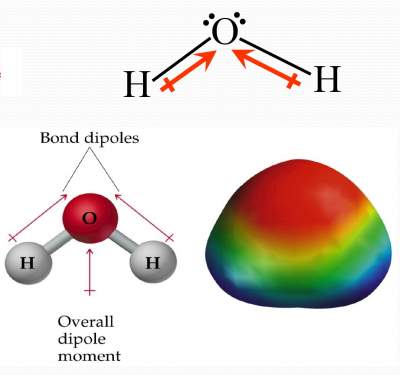

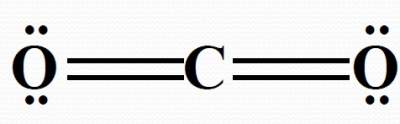
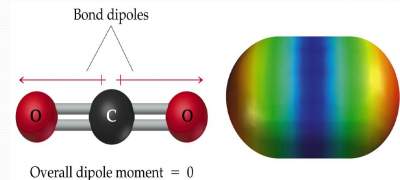
| In a H2O molecule; # e domains around O = 4; hence not linear. Bond dipoles DO NOT cancel each other. So, H2O is a POLAR molecule. |
 |
Check your answers here:
SolutionsCheck your answers here:
Solutions