The Isoelectronic Series
All members of an isoelectronic series have the same number of electrons.
Example:
O2−, F−, Na+, Mg2+, Al3+ (Having all 10 electrons)
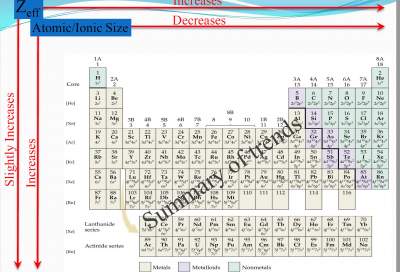
As the nuclear charge increases in an isoelectronic series, ions become smaller:
O2− > F− > Na+ > Mg2+ > Al3+
Exercise-2 on Comparing Sizes of Atoms and Ions of an Isoelectronic Series
Exercise-2 on Cations and Anions of an Isoelectronic Series
Check your answers here:
Solution to the Exercise-2 on Cations and Anions of an Isoelectronic Series
Exercise-3 on Comparing Sizes of Atoms and Ions of an Isoelectronic Series
Exercise-3 on Cations and Anions of an Isoelectronic Series
Check your answers here:
Solution to the Exercise-3 on Cations and Anions of an Isoelectronic Series
For more details, please contact me here.
Date of last modification: 2021
