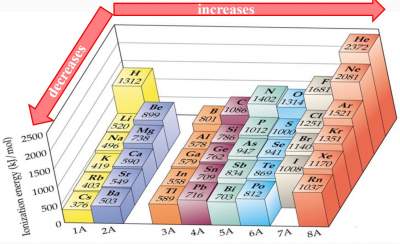
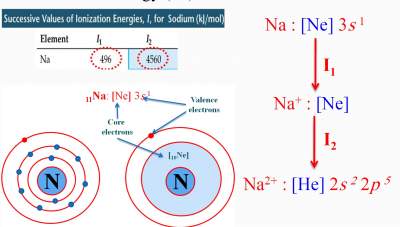
| As we move across a period, Zeff increases. Therefore, it becomes more difficult to remove an electron, i.e., more energy is required to remove an electron from the atom (The rule of Zeff) |  |
| As the principle quantum number (n) increases (by moving down a group), the distance of the outermost electron from the nucleus becomes larger, its attraction to the nucleus decreases, it becomes easier to remove an electron from the outermost orbitals. (The rule of n) | 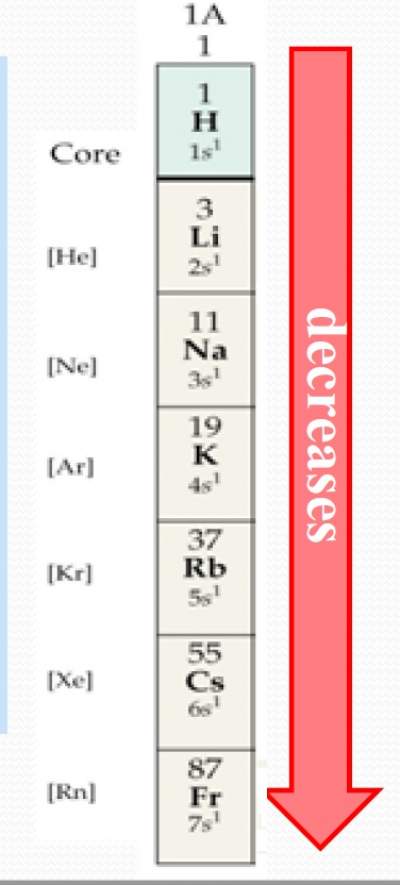 |