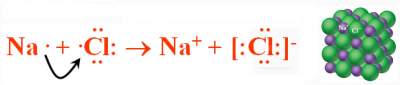Introduction to Ionic Bonding
Introduction
Cations and anions are formed in such a way that the "Octet Rule" is satisfied.
Example:
Na(s) + ½Cl2 → NaCl(s)
ΔH°r = − 410.9 Kj (highly exothermic)
Both Na and Cl in NaCl have inert gas configuration.
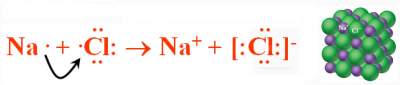
Energetics of Ionic Bond Formation
Definition: Lattice energy is the energy required to completely separate an ionic solid into its gaseous ions. E1 = κ(Q1Q2)/d
Where: κ is a constant = 8.99 x 109 J·m/C2,
Q1 and Q2 are the charges on the ions,
d is the distance between ions.
Lattice energy depends on:
- Charges on the ions (Q1 and Q2)
- Sizes of the ions (d)
Lattice energy increases as:
- Charges on the ions increase
- The distance between the ions decreases
Exercise: Energetics of Ionic Bond Formation
Exercise: Energetics of Ionic Bond Formation
Check your answers here:
Solutions
For more details, please contact me here.