Introduction to the VSEPR Model
Introduction
"The best arrangement of a given number of electron domainsis the one that minimizes the repulsions among them".
Principles:
- Electrons adopt an arrangement in space to minimize e−e repulsion.
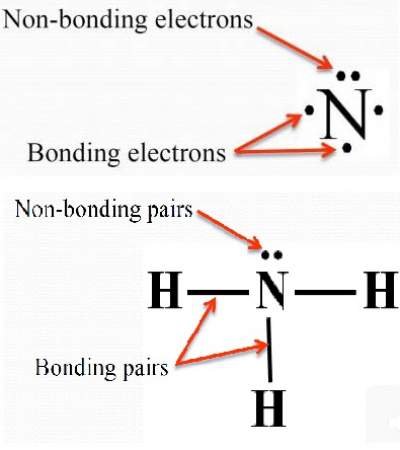
- When considering the geometry about the central atom, all electrons (bonding and non-bonding pairs) are considered.
- When naming the molecular geometry, we focus only onthe positions of the atoms.
According to the VSEPR theory, Geometry of a molecule is determined based on the number of electron domains around the central atom of a molecule, called Electron-Domain Geometry.
Types of Electron Domains
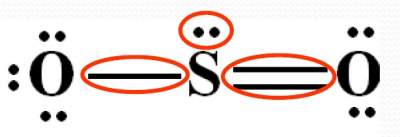
Example SO2
As shown below, the central atom (S) has three (3) electron domains: (one nonbonding pair, one single bond & one double bond)
For more details, please contact me here.
Date of last modification: 2021

