Introduction to Acid-Base Reactions
Acid-Base Reactions
Water (H2O) is made of H+ and OH− ions.
Remark:
H+ ions are H atoms that lost ONE electron leaving behind just a proton.
This explains why H+ ions are often called "protons".
Acids
Substances that ionize in water to form Hydrogen ions (H+), so that the total [H+] concentration in water increases.
Examples: HCl, HNO3, H2SO4
- If acids produce ONE H+ ion if dissolved in water. These are called "Monoprotic Acids" such as HNO3
- If they ionize to TWO H+ ions, they are called "Diprotic Acids" such as H2SO4
- If they produce MORE H+ ions, they are called "Polyprotic Acid" such as H3PO4
Bases
Substances that ionize in water to form hydroxide ions (OH−), so that the total [OH− ] concentration in water increases.
Bases also accept (react with) H+ ions.
Examples: NaOH, KOH, Ca(OH)2
Remark:
Some bases do not contain (OH−), but can still accept (H+) ions. They are also considered bases such as NH3
H2O + NH3 → NH4+ + OH−
Acids and Bases are grouped as follows:
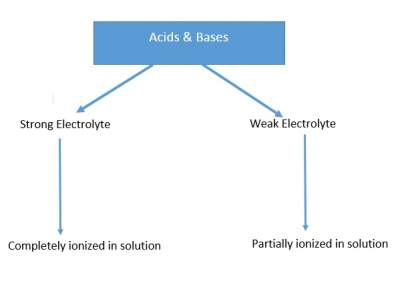
Strong Acids
HCl, HBr, HClO3, HI, HF is a weak acid, HNO3, H2SO4
Remark:
Because of this short list, most acids are weak.
Strong Bases
LiOH, NaOH, KOH, RbOH, CsOH, CaOH, SrOH2, BaOH2
Remark:
These are all soluble in water. NH3 is a weak base.
To identify an electrolyte as a strong, weak or non-electrolyte? We follow the following:
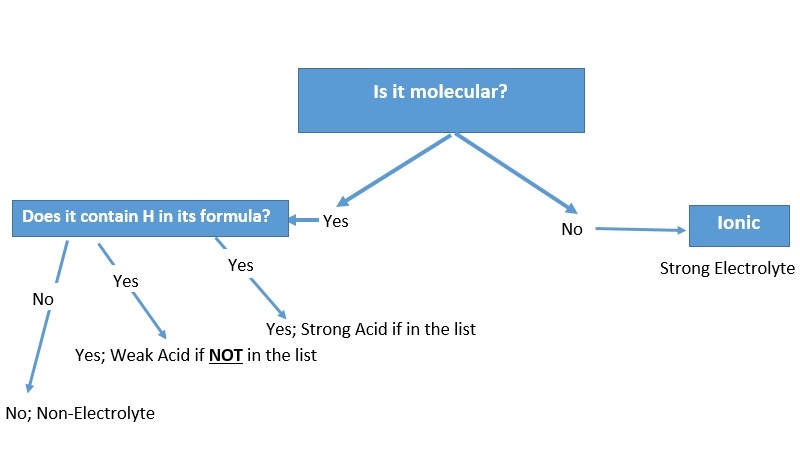
For more details, please contact me here.
Date of last modification: Summer , 2019

