Exercise on Resonance Lewis Structures: Solutions
Question: SO3 has three resonance Lewis structures.
Draw these structures and compare the S−O bond length in SO3 to a normal S−O single and S=O double bond lengths.
Solution:
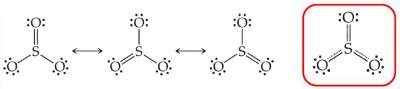
Because of resonance, the S−O bond in SO3 is neither single nor double bond.
It is shorter than S−O single bond, but longer than S=O double bond.
For more details, please contact me here.
Date of last modification: Summer , 2019
