Exercise on Lewis Structures and Formula Charge: Solutions
Question: CO2 has the following two Lewis Structures.
Use Formula Charge calculations to decide on the most stable of them.

Solution:
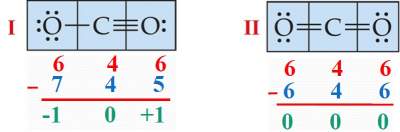
Structure I is NOT possible Since O, a high electronegative element, CANNOT have a POSITIVE Formal Charge.
However, Structure II is the most possible; most stable
For more details, please contact me here.
Date of last modification: Summer , 2019

