Exercise with Solution: Drawing Lewis Structures-1
Questions:
Question 1: Draw the Lewis Structure of PCl3
Question 2: Add the valence electrons of all atoms in the compound.
Solution:

(1 × 5) + (3 × 7) = 26 electrons.
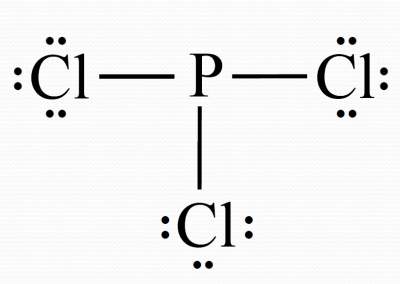
Write symbols for the atoms and show which atoms are connected to which, and connect them with a single bond.

Complete the octet for atoms bonded to the central atom.
We used 24 electrons, leaving 2 electrons un-located.
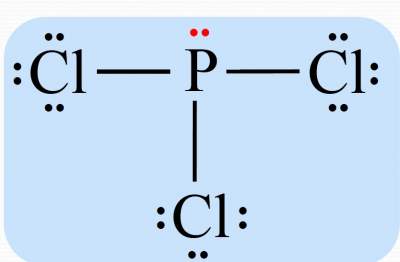
Place leftover electrons on the central atom.
For more details, please contact me here.



