Empirical Formula from Analysis
Introduction
Example
- 1 molecule of H2O contains 2 H atoms & 1 O atom.
- 1 mol of H2O contains 2 mol of H atoms and 1 mol of O atom.
- Hence, if the number of moles of the atoms are known, number of moles of their molecule can be determined.
The procedure is shown below:
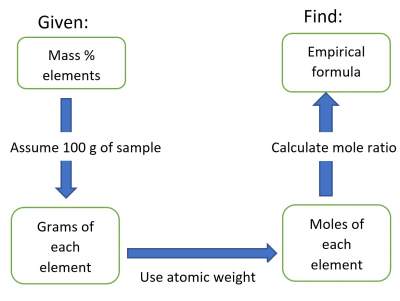
Example: Hg reacts with Cl forming a compound that is 73.9 % Hg and 26.1% Cl by mass.
Question 1: What is the empirical formula of the compound? (Note: Atomic weights of Hg and Cl are 200.6 and 35.5, respectively).
Solution:
- These two elements form ONE compound, whose formula is to be determined.
- The question is about the Empirical Formula; the simplest formula of this compound Procedure
- Assume 100 g sample, this contains: 73.9 g Hg & 26.1 g Cl
- Use the atomic weights of Hg and Cl, to calculate their number of moles: For Hg: # moles = (73.9 / 200.6) = 0.37
- For Cl: # moles = (26.1 / 35.5) = 0.74
- Calculate the molar ratio as follows: Hg : Cl (0.37 : 0.74)
- Divide all numbers by the smallest # of moles Hg : Cl (1 : 2)
Hence, the empirical formula is HgCl2
Question 2: What is the relation between: a "Molecular Formula" and an "Empirical Formula"?
Answer:
Remember the following:
To find the empirical formula of the molecular formula, C6H12O6, we need to divide by the common number, which is 6 in this example, and we obain: CH2O
So, a molecular formula is an actual formula of the molecule, and the empirical formula is a simpler (abstracted) form.
Similarly, 180 g/mol (molecular formula weight), and then dividing it by 6 (the common number), we obtain 30 g/mol (empirical formula weight).
Hence, knowing the empirical formula, its weight and the molecular formula weight will let us determine the molecular formula.
For more details, please contact me here.
Date of last modification: Summer , 2019
