Electron-Domain Geometries
Introduction
How can an electron-domain geometry be determined?
Steps:
- Draw the Lewis structure.
- Count the total number of electron pairs (domains)around the central atom.
- Arrange the electron pairs (domains) in one of the five (5)geometries to minimize e−e repulsion.
Example: NH3 Molecule
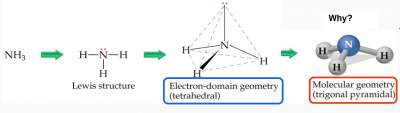
What is the difference between:
- Electron-domain geometry
- Molecular geometry
This is attributed to the difference between bonding and non-bonding electrons
Electron-Domain Geometry: depends on the number of electron domains around the central atom.
Molecular Geometry: depends on the type of electron domains around the central atom; so that non-bonding pairs will no be extended in space.
The effect of difference in type of electron domain on bond angle:
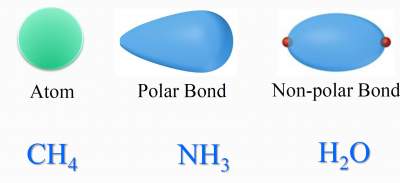
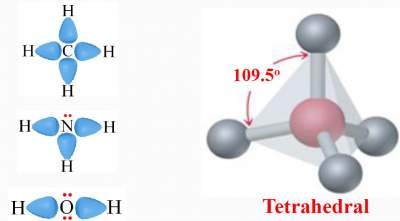
Based on the # of electron domains around each of the central atoms, these molecules should adopt the tetrahedral
geometry with a typical angle of 109.5° .
For more details, please contact me here.


