Covalent Bonding and Orbital Overlap
Disadvantages of VSEPR theory
- Does not explain why a bond forms.
- Does not tell which orbitals are involved in bonding?
Hence, the need of Valence Bond Theory where
"Bonds form when orbitals on atoms overlap".
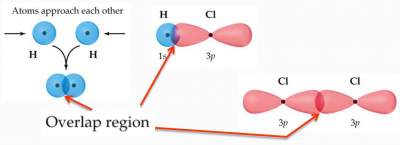
- As two atoms approach each other their atomic orbitals overlap.
- As the amount of overlap increases, the energy of the interaction decreases.
- At some distance the minimum energy is reached. The minimum energy corresponds to the bonding distance (bond length).
- At some distance the minimum energy is reached. The minimum energy corresponds to the bonding distance (bond length).
- As the two atoms get closer, their nuclei begin to repel and the energy increases.
Example: Overalp of orbitals explains the mechanism of bond formation
For more details, please contact me here.
Date of last modification: Summer , 2019
