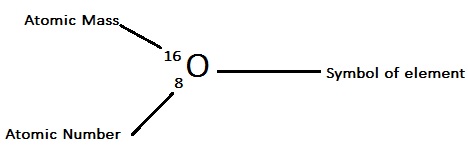Atomic Number and Atomic Mass
Introduction
The way elements differ from each other as illustrated below:
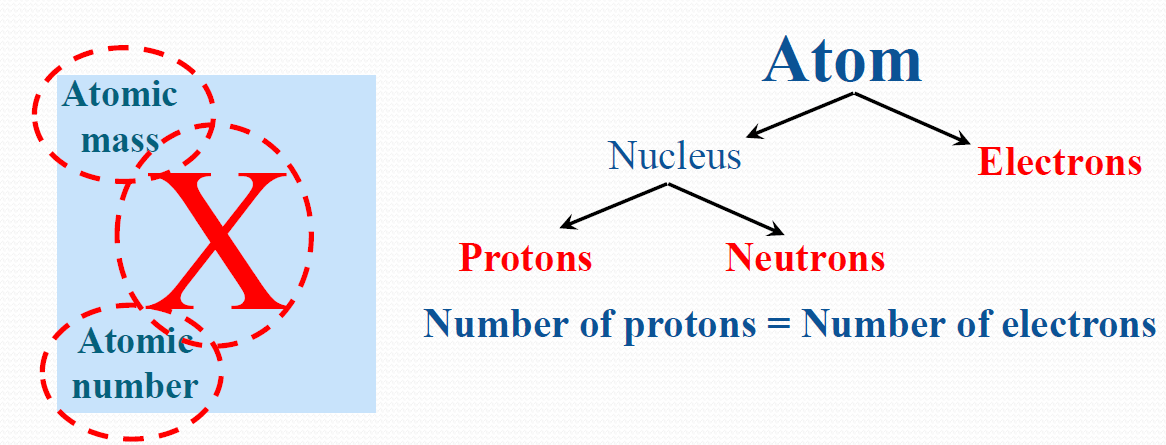
- Atomic Number = Number of electrons = Number of Protons
- Atomic Mass = Number of Protons + Number of Neutrons
Example
Example 1: Here is an example of the atomic mass and atomic number of Oxygen (O).
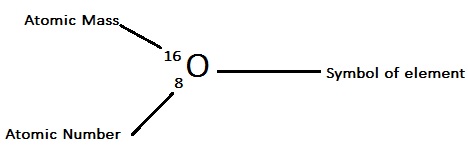
Exercise: Finding number of protons, neutrons, and electrons
Exercise-1 on the Number of Atoms
Check your answers here:
Solution to Exercise-1 on Number of Atoms
Isotopes
These are atoms of a given element that differ in the number of neutrons and consequently in mass.
Example: Oxygne isotopes as shown below where each one is called a nuclide.
16O, 17O, 18O
where 18O is the most abundant (99.762% natural abundance).
Exercise: Isotopes of Magnesium
Exercise-1 on Isotopes
Check your answers here:
Solution to Exercise-1 on Isotopes
For more details, please contact me here.
Date of last modification: Summer , 2019